-
Research area
- Biochemicals
- Blood and Biospecimens
- Cell biology
- Environmental
- Flow Cytometry
- Forensic Science
- Genomics
- Immunology
- Labware
- Microbiology
- Pathology
- Transplantation
429 Too Many Requests 429 Too Many Requests
nginx - Suppliers
- About us
- Resources
- Events
- Support
- Lab Services
Legenda image
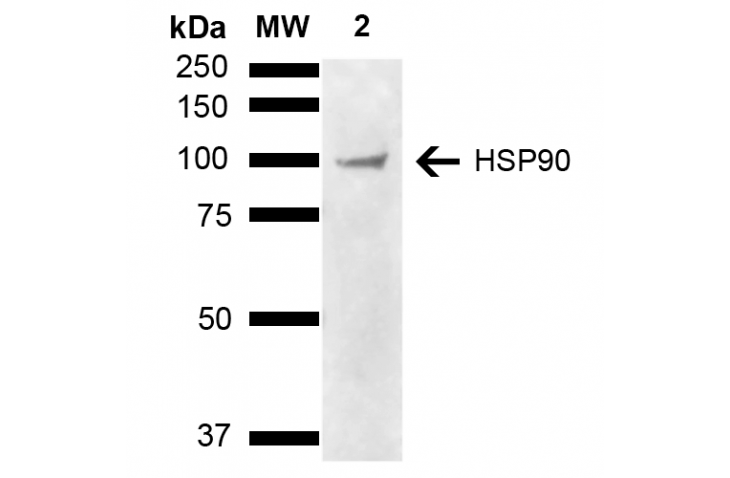
Llama Anti-HSP90 alpha/beta Antibody used in WB on Cervical cancer cell line (HeLa) lysate (SPC-745)
Product description
HSP90 is an abundantly and ubiquitously expressed heat shock protein. It is understood to exist in two principal forms ? and ?, which share 85% sequence amino acid homology. The two isoforms of HSP90 are expressed in the cytosolic compartment (1). Despite the similarities, HSP90? exists predominantly as a homodimer while HSP90? exists mainly as a monomer (2). From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (3-6). Furthermore, HSP90 is highly conserved between species; having 60% and 78% amino acid similarity between mammalian and the corresponding yeast and Drosophila proteins, respectively.
HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. Despite its label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the HSP90-regulated proteins that have been discovered to date are involved in cell signaling (7-8). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase (5). When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation.
In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immunoadsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (9). For more information visit our HSP90 Scientific Resource Guide at http://www.HSP90.ca.
Specifications
Applications
WB, ELISA
Host
Llama
Clonality
Polyclonal
Isotype
IgG1, IgG2, IgG3
Supplier
Stressmarq
Shipping & storage
Shipping condition
Blue Ice
Storage temperature
2-8°C
Do you have any questions about this product?
Order your product by email
Productname
Llama Anti-Human HSP90 alpha/beta Polyclonal
SPC-745D-A390
By filling out this form, you are placing an order by e-mail. You will receive an order confirmation within one working day. The order cannot be modified after receipt of the order confirmation.
Request a sample
Productname
Llama Anti-Human HSP90 alpha/beta Polyclonal
SPC-745D-A390
By filling out this form, you request a sample. You will receive an order confirmation within one working day. The order cannot be modified after receipt of the order confirmation.
Are you looking for specific products, alternatives or documentation?