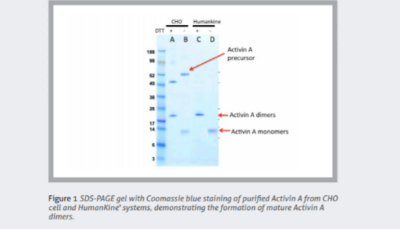
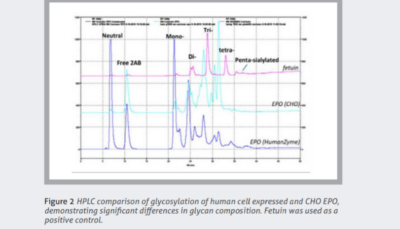
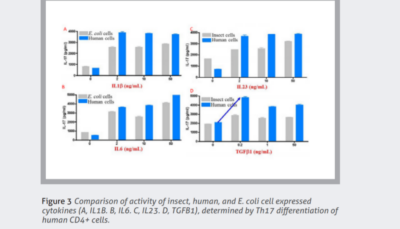
Cytokines and growth factors are essential to advanced therapies. Unlike traditional pharmacologics, cell and gene therapies require the expansion and maintenance of living cells. These recombinant proteins coordinate and sustain these processes.
“FOR HUMANS...BY HUMAN CELLS”
With the rise in clinical trials, there is a greater demand for safe, pure, and potent recombinant proteins. Xeno-free and animal component-free, Proteintech’s HumanKine recombinant proteins are ISO 13485 certified and GMP-compliant, making them ideal for clinical applications.
Proteintech Group manufactures RUO and GMP-grade HumanKine recombinant proteins using a human expression system.
Recombinant proteins created in HEK293 cells using animal-free components offers the following advantages when compared with proteins manufactured using bacterial based platforms:
- High bioactivity & stability
- Lot-to-lot consistency
- Native human conformation & post-translational modifications
- Endotoxin-free, xeno-free & tag-free