All scientists need to obtain convincing images to support the biological phenomenon they are studying. The main question is how to choose the best microscope setup to answer the experimental question in the most efficient manner.
FLUORESCENCE MICROSCOPY: WIDEFIELD VERSUS CONFOCAL MICROSCOPY
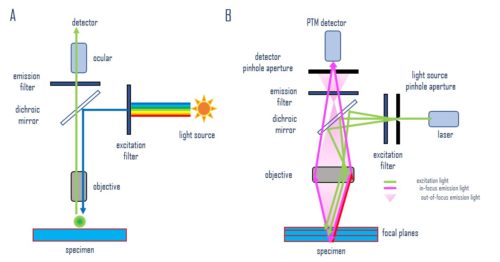
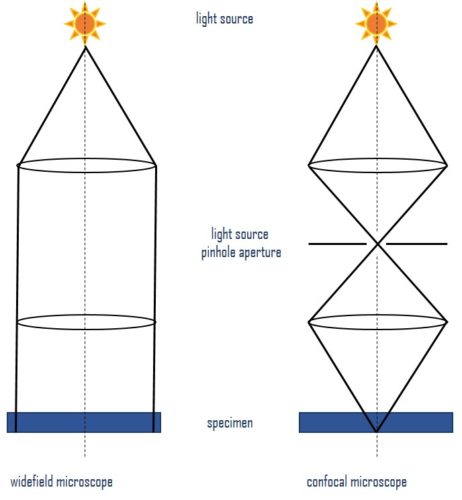
Fluorescence microscopy is an imaging technique that uses fluorescent probes, dyes, or proteins (fluorophores) for sample labeling to generate an image. Which microscope you choose will depend on the individual experiment. Widefield microscopes are more robust and provide a general picture quickly. While confocal microscopes should be used for thicker samples, or higher-resolution pictures (Figure 1).
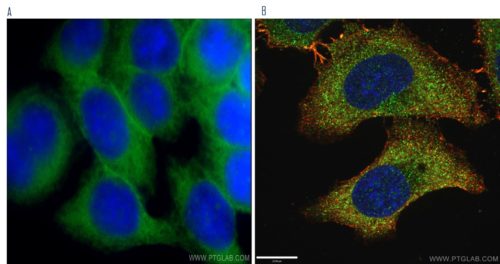
Figure 1.
A) Immunofluorescent analysis of HeLa cells using SLC2A1, GLUT1 antibody (21829- 1-AP) at a dilution of 1:50 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG(H+L) by widefield microscope.
B) Immunofluorescent analysis of (-20°C Ethanol) HepG2 cells fixed using LAMP1 antibody (55273-1-AP) at a dilution of 1:50 and Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG(H+L) by confocal microscope. DAPI was used as the nuclear counterstain.