Watch the recorded webinar 'Improving the accuracy of cfDNA-based assays with DNA Cleared Plasma' here.
Liquid biopsy assays that detect and quantify cell free DNA (cfDNA) have emerged as a powerful tool for the sensitive detection of tumors and treatment monitoring. However, accurate quantitation of cfDNA is challenged by both the low abundance of cfDNA in plasma and the complex, multi-component nature of the plasma matrix—simple aqueous buffers and synthetic matrices do not adequately replicate the behavior of plasma when creating controls for cfDNA extraction using spiked-in DNA fragments.
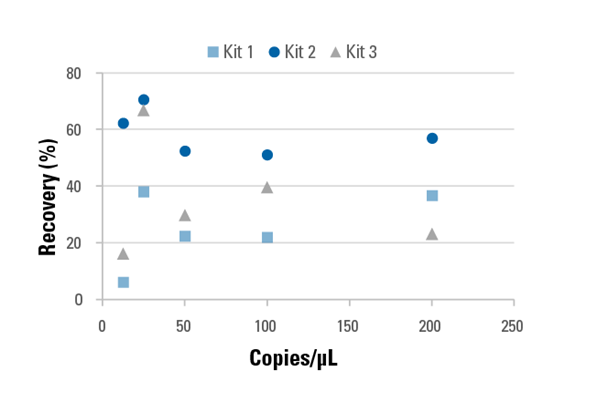
In this recorded webinar, SBI shows how their DNA Cleared Plasma is a better control matrix for cfDNA assays than synthetic matrices, especially for validation of plasma-based cfDNA assays. The composition of DNA Cleared Plasma is virtually identical to natural plasma but without any detectable free DNA, making it a unique and effective option for replicating real sample conditions during assay development, leading to more accurate and precise assays.
