Combining the principles of developmental biology with stem cell culture techniques to grow biological tissues in a dish
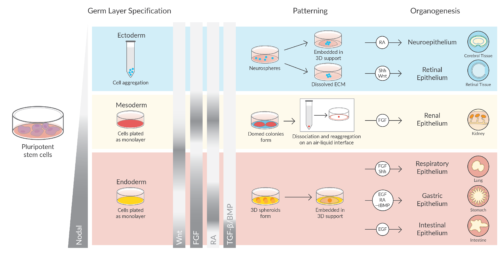
Researchers have devised methods to generate stable, physiologically relevant miniature organ models that simulate brain, liver, thymus, thyroid, lung, pancreas, and heart tissue. In addition to providing a detailed view of how organs form and grow, these models—called organoids—are used for drug discovery and toxicology research, infectious disease modeling, and tissue engineering/gene editing for regenerative medicine, providing new pursuits for developing personalized therapeutics from individualized tissues.
Organoids are tiny, three-dimensional cultures that are derived from tissue or pluripotent stem cells. Using the knowledge gained from maintaining stem cell populations, the cells are cultured in an environment that allows them to follow their own genetic instructions to self-organize into tissue with some organ-like functionality that contains a self-renewing stem cell population. Self-assembly and differentiation are the result of instructive signaling cues given to the cells by the extracellular matrix, the culture medium, and, by the cell types present in the organoids themselves—once the structure assembles.